Chemical Equations
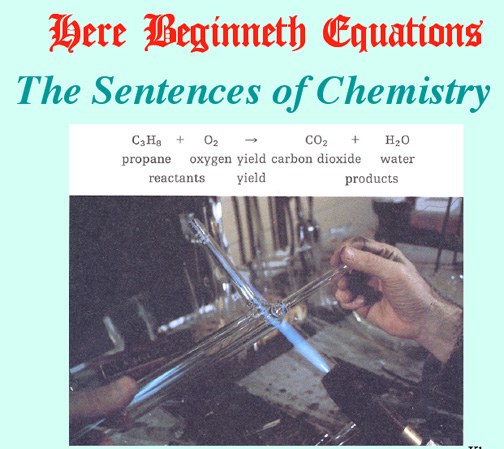
Demos to Perform:
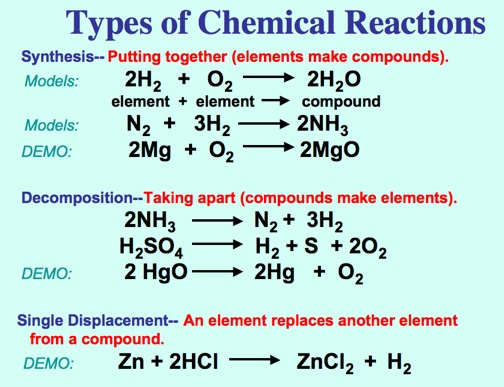
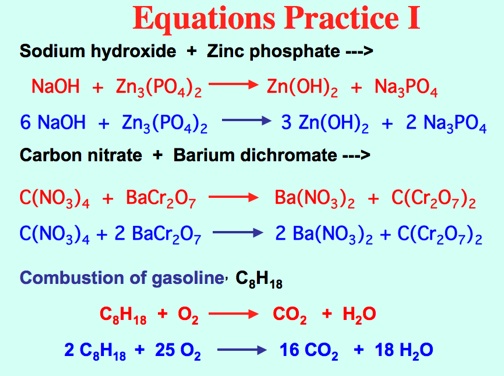
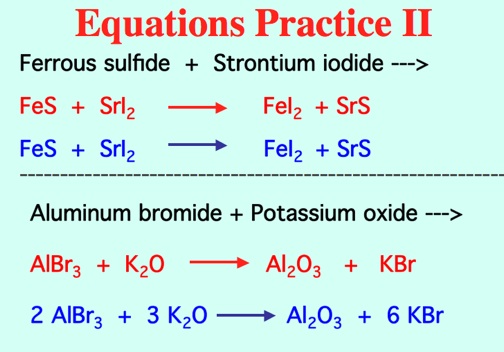
Types of Reactions:
Synthesis:
Mg + O2 ---> 2MgO
Decomposition:
HgO ---> Hg + O2
Single Displacement:
Zn + 2HCl ---> ZnCl2 + H2
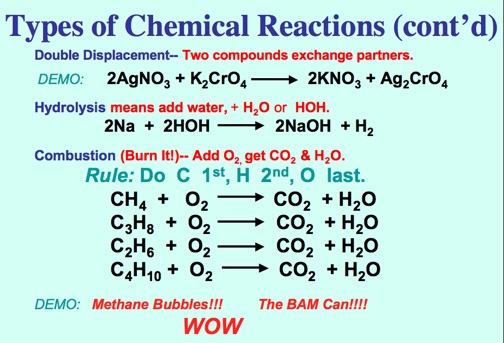
Double Displacement:
2AgNO3 + K2CrO4 ---> 2KNO3 + Ag2CrO4
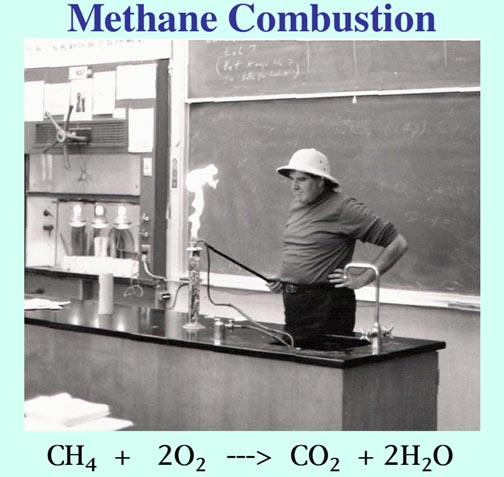
Combustion:
CH4 + 2O2 ---> CO2 + 2H2O