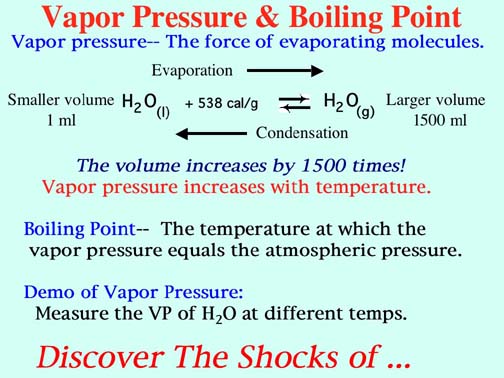
Equilibrium
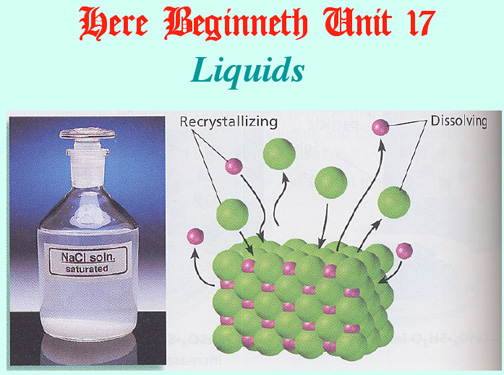
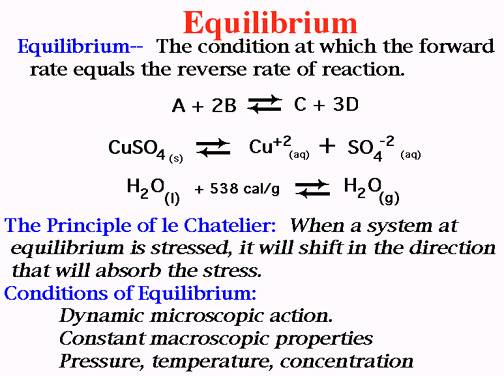
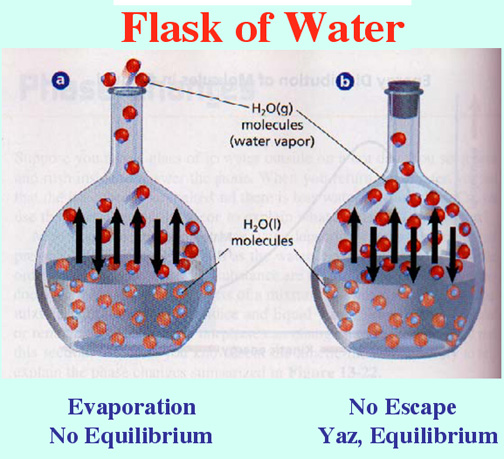
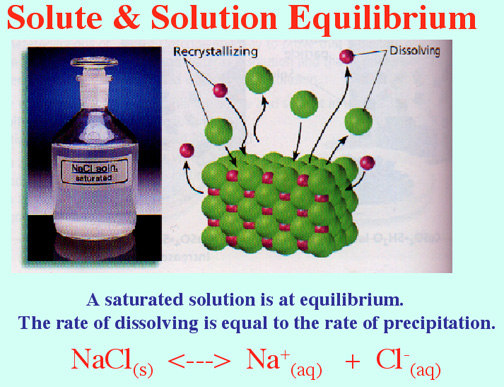
Equilibrium



Gas Model demos, Video:

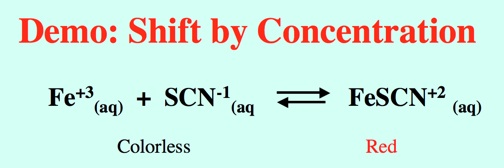

Demo:
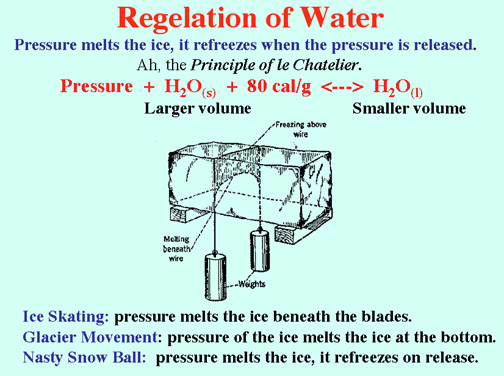

Demos:


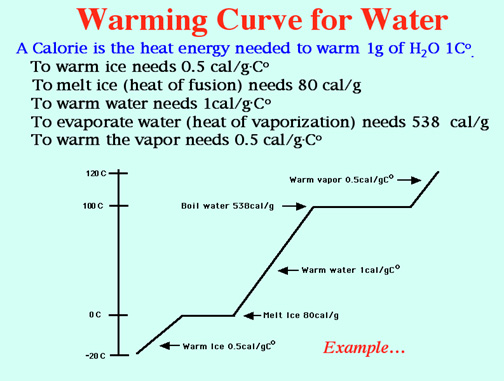
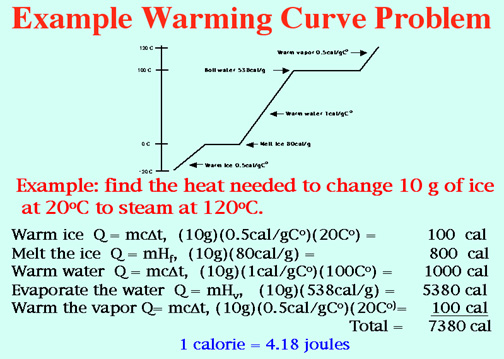


Super Heated Steam is awesome! Video:
Freezing by Boiling!

Superheated water (200oC) under high pressure (30kg/cm2) expands explosively when the pressure is released. The heater rockets 150 meters high! Video:
We can have a superheated liquid at room temperature (20oC) like the CO2 in a fire extinguisher. Liquid CO2 at 20oC has a vapor pressure of 100 atmospheres (100 kg/cm2! To contain that we need a strong steel container. If the valve is broken off, the awesome flashing of liquid to gas makes the cylinder into a rocket that can penetrate a wall! Video:


After steam cleaning, the tanker was sealed up.
The steam condensed to water leaving low pressure inside!
1 kg/cm2!
Crushing cans and steel barrels by condensing steam. WOW! The steam condenses about 1500 times going from Gas to Liquid. This huge reduction of pressure inside the can allows atmospheric pressure ( 1 kg/cm2) to smash the can and barrel. Video:


Duckie's head is cooled by the evaporation of water in the wet fabric. So the vapor pressure is lower at the top. The vapor pressure in the warm rump is greater causing the fluid to rise up in the neck of Duckie. This causes Duckie to tip over and dump the fluid back into his lower regions.
In the Franklin Love Meter, warm fingers (37oC) raise the vapor pressure causing the liquid to rise up and bubble at the top.
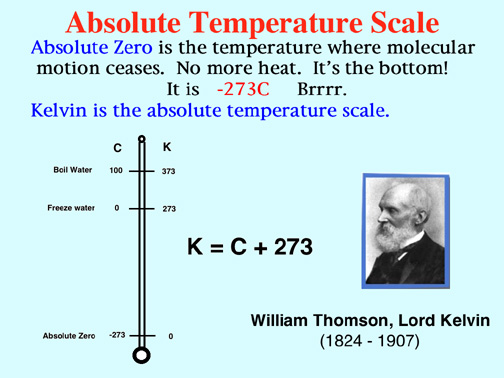
Demo: Determine Absolute Zero with the Charles' Law Apparatus.
Boiling nitrogen demos, Video:
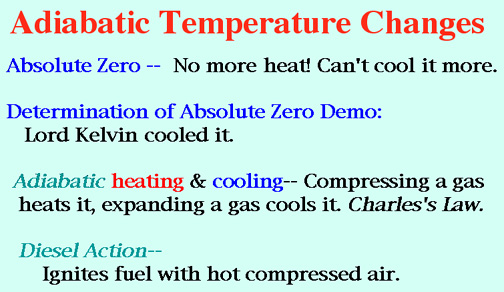
Demos:
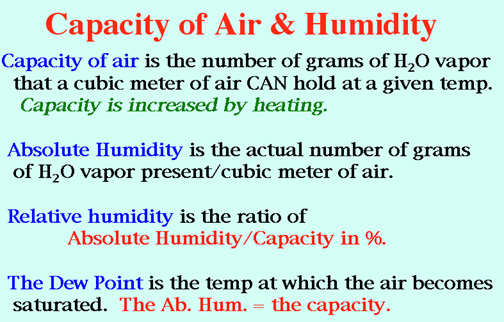




Demo:
The Sling Psychrometer Measures Relative Humidity



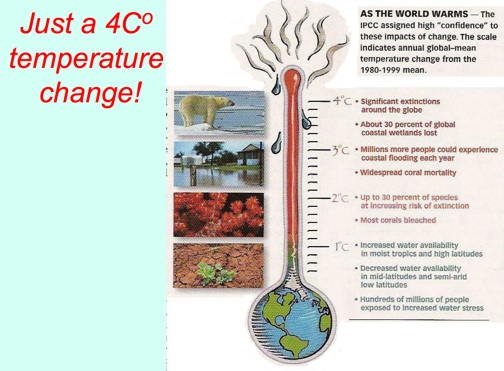
Just a 4Co Temperature Change!

Demos:


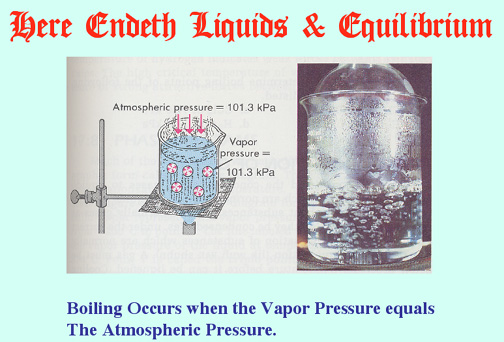
Here Endeth Equilibrium