Unit 23
Reaction Rates

Demos to Prepare:
Hot Ball + Gasoline
Boil water in a paper cup
Burn Sugar with ashes catalyst
Spontaneous Combustion
Dust Explosion! Powder in can. BAM!
Make CO2 with Vinegar & Soda, extinguish Candle, Burn Magnesium Ribbon
Ignite methane fumes in tube
Standing waves in 2-meter tube. Whoooshhhh!
Big oxidation-- Put an M&M into boiling KClO3 WOW!
The burning of the Hindenburg. Video:
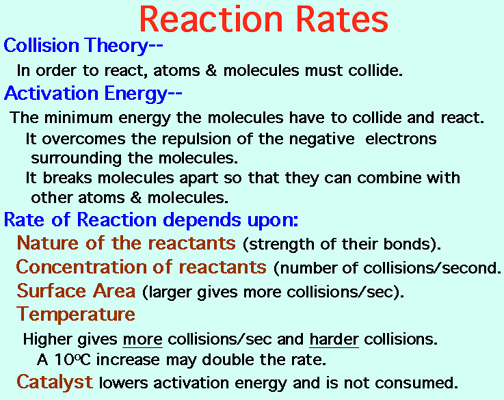
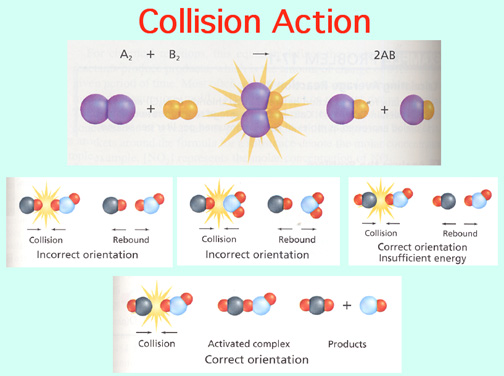
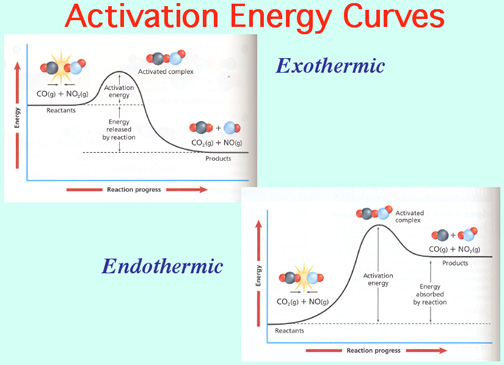
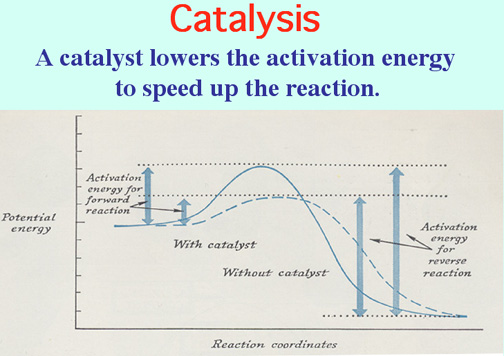
Catalysts lower the Activation Energy to speed up a reaction! Video:
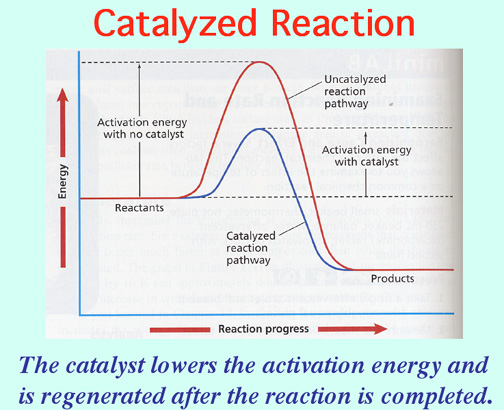

Cops and Catalysts! Video
Burning magnesium produces so much heat that it decomposes carbon dioxide to produce oxygen to keep the magnesium burning! WOW! Video:
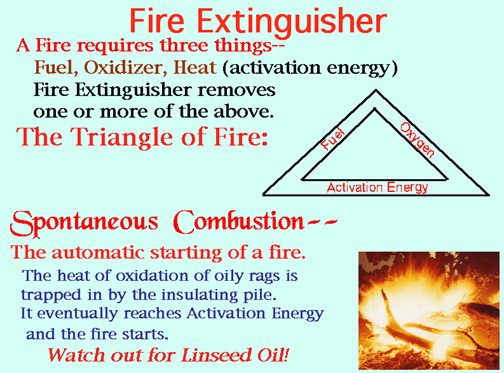
WARNING! Kitchen oil fire. NEVER hose an oil fire with a stream of water! It will blast burning oil all over! Use a dry powder extinguisher or a damp towel to smother the fire. Fine spray water is okay, too. Video:
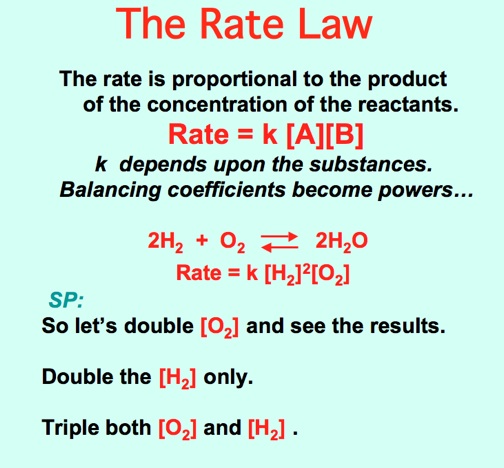
The Carbide Canon demonstrates a fast reaction rate. Video:
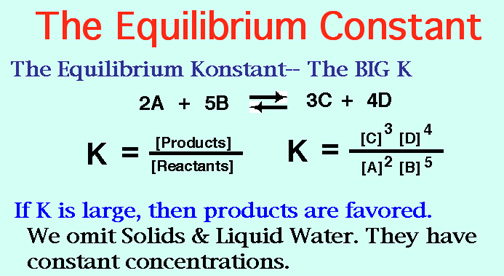
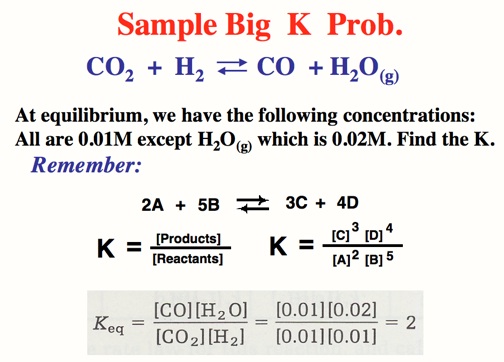
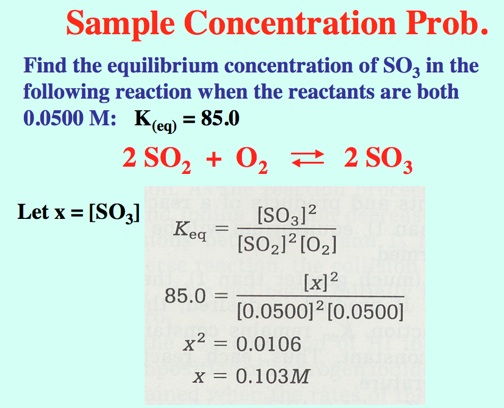
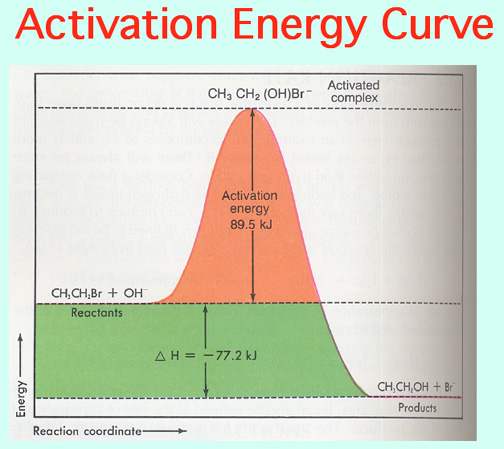
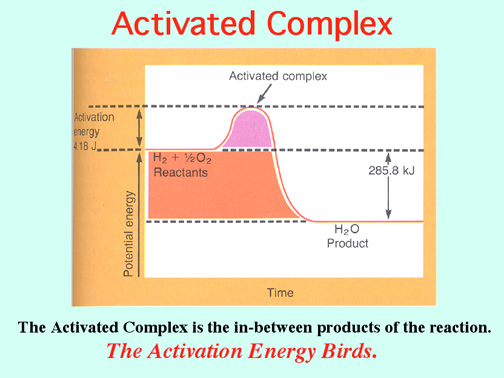
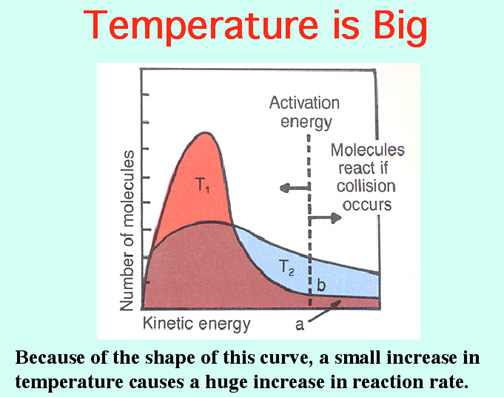
The Alkali Metals roar into action with low Activation Energies, Video:

Here Endeth Rates of Reactions