THE BLACK BANANA

The Dehydration of Sugar is very entertaining even if it is somewhat dangerous.
The purpose of this reaction is to practice Alchemy, no, no, it's to demonstrate that a compound is a substance that can be broken down into simpler substances.
Also we shall show that a chemical reaction causes observable changes such as color change, temperature change, odor change, and gases formed.
New substances will be formed as old ones are destroyed.
Place a 100 ml beaker onto a brick. This reaction produces lots of heat!
Have a beaker grabber handy to prevent the beaker from overturning.
With Goggles ON, pour 40 ml of 18M H2SO4 into the 100 ml beaker.
Carefully stir in enough sugar to make a pasty mass.
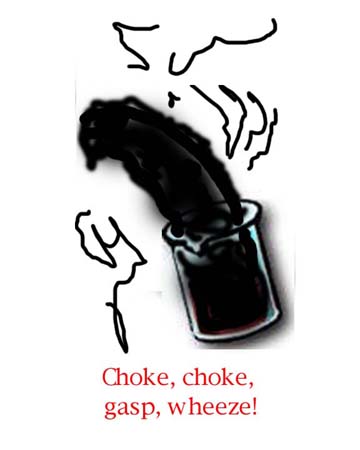
Note the color changes as the reaction proceeds. Soon SO2 choking, poisonous fumes will be liberated. Avoid breathing them!
The sugar is broken down into Carbon as Steam and Sulfur Dioxide gases are released. The Carbon forms the "Black Banana" which expands right out of the beaker. It usually curves over from whence the "Banana" term comes.
Do Not handle the "banana" as it still contains Sulfuric Acid!
To dispose of it, use an implement to pry it out into a trash can. Carefully wash out the beaker which still contains acid.